Deupirfenidone 825 mg TID slowed lung function decline in people with idiopathic pulmonary fibrosis (IPF) to the range expected of healthy older adults over 6 months; new, preliminary open-label extension data support durability of this treatment effect over at least 52 weeks
Deupirfenidone 825 mg TID demonstrated a statistically significant benefit compared to placebo in delaying IPF progression
Detailed safety analysis underscores favorable tolerability profile for deupirfenidone
"The ELEVATE IPF trial is one of the most promising Phase 2 studies we've seen in IPF in recent years," said
Data presented from
In addition to these findings, deupirfenidone 825 mg TID also demonstrated a statistically significant benefit in delaying time to IPF progression4 compared to placebo (hazard ratio = 0.439; p=0.0023), further supporting the clinical relevance of the treatment effect.
Importantly, the rate of FVC decline observed over 26 weeks with deupirfenidone 825 mg TID (-21.5 mL) was similar to the expected natural decline in lung function in healthy older adults (approximately -15.0 mL to -25.0 mL).5,6 Furthermore, preliminary data from the ongoing open-label extension (OLE) study suggest that this treatment effect is durable out to at least 52 weeks. As of
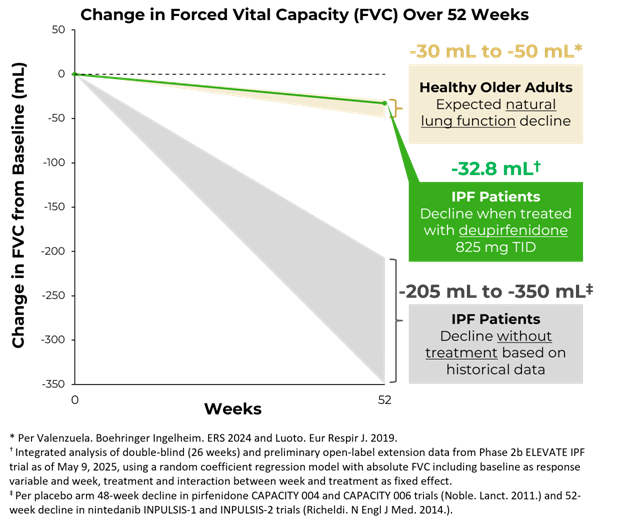
These results are further supported by preliminary pharmacokinetic (PK) data, which underscore the differentiated profile of deupirfenidone. Compared to pirfenidone 801 mg TID, deupirfenidone 825 mg TID resulted in an approximately 50% increase in drug exposure. Notably, the dramatically increased drug exposure did not result in an increase in tolerability challenges, suggesting that the deuterated structure of deupirfenidone may overcome the dose-limiting adverse events associated with pirfenidone.
"The IPF community has long needed therapies that can provide meaningful efficacy without compromising tolerability," said
Deupirfenidone was well tolerated at both doses studied. Safety analyses included identification of the 16 most common treatment-emergent adverse events (TEAEs), defined as occurring in more than 5% of participants in at least one treatment group, and characterized the arm with the highest relative incidence of each of these 16 TEAEs. The pirfenidone 801 mg treatment group had the highest relative incidence for 9 of these TEAEs, followed by deupirfenidone 825 mg (5), placebo (2), and deupirfenidone 550 mg (0).
"The results of the ELEVATE IPF trial demonstrate the potential for deupirfenidone to address the persistent suboptimal efficacy offered by current standard-of-care treatments for IPF, without sacrificing tolerability,"
About the ELEVATE IPF Trial
The Phase 2b ELEVATE IPF trial was a global, randomized, double-blind, active- and placebo-controlled, dose-ranging trial designed to evaluate the efficacy, tolerability, safety and dosing regimen of deupirfenidone (LYT-100) in patients with IPF compared to placebo. 257 participants were randomized in a ratio of 1:1:1:1 to receive either 550 mg of deupirfenidone, 825 mg of deupirfenidone, 801 mg pirfenidone or placebo three times a day (TID) for 26 weeks. Participants who completed the trial had the option to enroll in an open-label extension, which is ongoing.
The primary endpoint of the trial was the rate of decline in Forced Vital Capacity (FVC) for the combined deupirfenidone arms versus placebo over the 26-week treatment period. FVC is a measure of the maximum amount of air (in mL) that an individual can forcibly exhale after fully inhaling. It is a standard measurement in clinical trials for IPF and is used to assess disease progression as well as to predict mortality.
A prespecified Bayesian analysis was utilized to assess the primary endpoint and provided a posterior probability, which is the probability of superior efficacy for deupirfenidone compared to placebo. This also allowed for augmentation of the placebo arm with placebo data from historical IPF trials. This approach enabled a more patient-centric clinical trial design by minimizing the number of trial participants exposed to placebo - a key consideration since IPF is progressive and fatal - while delivering a robust, placebo-controlled dataset.
About Deupirfenidone (LYT-100)
Deupirfenidone (LYT-100) is an investigational therapy in development as a potential new standard of care (SOC) for the treatment of idiopathic pulmonary fibrosis (IPF). It is a deuterated form of pirfenidone, which - along with nintedanib - is one of the two FDA-approved treatments for IPF. Despite achieving blockbuster status, the current SOC treatments only modestly slow lung function decline, with tolerability limiting the ability to achieve higher doses. This results in suboptimal efficacy, reduced patient uptake, and poor adherence - all due to a tolerability ceiling that prevents dosing levels that could significantly improve patient outcomes.
Deupirfenidone may overcome these limitations. In the global Phase 2b ELEVATE IPF trial, deupirfenidone demonstrated the potential to stabilize lung function decline over at least 26 weeks as a monotherapy while maintaining safety and tolerability - a result not previously achieved by other investigational or marketed IPF therapies to the Company's knowledge. These findings support the potential for deupirfenidone to offer a meaningful advance for patients living with this progressive and life-limiting disease. Beyond IPF, deupirfenidone may also address multiple underserved fibrotic diseases, including progressive fibrosing interstitial lung diseases and other fibrotic conditions.
About Idiopathic Pulmonary Fibrosis (IPF)
Idiopathic Pulmonary Fibrosis (IPF) is a rare, progressive and fatal lung disease characterized by irreversible scarring of lung tissue. Median survival following diagnosis is estimated to be two to five years.8 IPF affects more than 230,000 people across
Although two therapies are approved to treat IPF, their use remains limited, and nearly three out of four people with IPF in
About
For more information, visit www.puretechhealth.com or connect with us on X (formerly Twitter) @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation those related to the deupirfenidone (LYT-100) development program and development plans, its potential benefits to patients, plans for discussions with regulatory authorities, the further development of the program, future presentation of additional data from the trial and our future prospects, developments and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks, uncertainties and other important factors that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, those risks, uncertainties and other important factors described under the caption "Risk Factors" in our Annual Report on Form 20-F for the year ended
References
1 Efficacy analysis used a random coefficient regression model with absolute FVC including baseline as response variable and week, treatment and interaction between week and treatment as fixed effect. The analysis was performed based on the predefined Full Analysis Set.
2 All p values are two-sided and have not been corrected for multiplicity.
3 Roche. (2014). Esbriet® (pirfenidone) prescribing information.
4 IPF progression was defined as a ≥5% decline in FVCpp or death.
5 FVC decline at 6 months was estimated assuming linear decline over time.
6 Valenzuela, C., Bonella, F., Moor, C., Weimann, G., Miede, C., Stowasser, S., & Maher, T. (2024, September). Decline in forced vital capacity (FVC) in subjects with idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF) compared with healthy references [Poster presentation].
7 Analysis conducted using the same methodology to assess rate of decline in FVC at 26 weeks from the double-blind portion of the trial (see reference 1)
8 Fisher, M., Nathan, S. D., Hill, C., Marshall, J.,
9 GlobalData. (n.d.). Epidemiology and market size search: EU5 =
10 Dempsey, T. M., Payne, S., Sangaralingham, L., Yao, X., Shah, N. D., & Limper, A. H. (2021). Adoption of the antifibrotic medications pirfenidone and nintedanib for patients with idiopathic pulmonary fibrosis. Annals of the
Contact:
Public Relations
publicrelations@puretechhealth.com
Investor Relations
+44 (0) 20 3727 1000
US Media
+1 609 578 7230
RNS may use your IP address to confirm compliance with the terms and conditions, to analyse how you engage with the information contained in this communication, and to share such analysis on an anonymised basis with others as part of our commercial services. For further information about how RNS and the
